Physicists found a way to make thermodynamics work in the quantum world
- Date:
- December 23, 2025
- Source:
- University of Basel
- Summary:
- More than 200 years ago, Count Rumford showed that heat isn’t a mysterious substance but something you can generate endlessly through motion. That insight laid the foundation for thermodynamics, the rules that govern energy, work, and disorder. Now, researchers at the University of Basel are pushing those rules into the strange realm of quantum physics, where the line between useful energy and random motion becomes blurry.
- Share:
In 1798, officer and physicist Benjamin Thompson (a.k.a. Count Rumford) made a simple but powerful observation while watching cannon barrels being drilled in Munich. The metal heated up continuously during the process, leading him to conclude that heat is not a physical substance. Instead, it can be produced endlessly through mechanical friction.
To test this idea, Rumford placed the cannon barrels in water and timed how long it took for the water to boil. His measurements showed that motion alone could generate large amounts of heat. Experiments like these laid the groundwork for thermodynamics in the 19th century. At first, this new field played a key role in the Industrial Revolution by explaining how heat could be converted efficiently into useful work, such as powering steam engines.
The Core Laws of Energy and Disorder
Today, the laws of thermodynamics are foundational knowledge for scientists. They state that in a closed system, the total amount of energy stays the same, whether it appears as heat or work. They also describe entropy, a measure of disorder, which never decreases over time.
While these principles hold true in everyday situations, problems arise when scientists try to apply them to extremely small systems governed by quantum physics. At that scale, familiar ideas about heat and work start to blur.
A Quantum Challenge to Classical Physics
Researchers at the University of Basel, led by Professor Patrick Potts, have developed a new approach to defining thermodynamic quantities for certain quantum systems. Their findings were recently published in the scientific journal Physical Review Letters.
"The problem we have with the thermodynamic description of quantum systems is that in such systems, everything is microscopic. This means that the distinction between work, which is useful macroscopic energy, and heat, or disordered microscopic motion, is no longer straightforward," doctoral student Aaron Daniel explains.
Laser Light in a Cavity
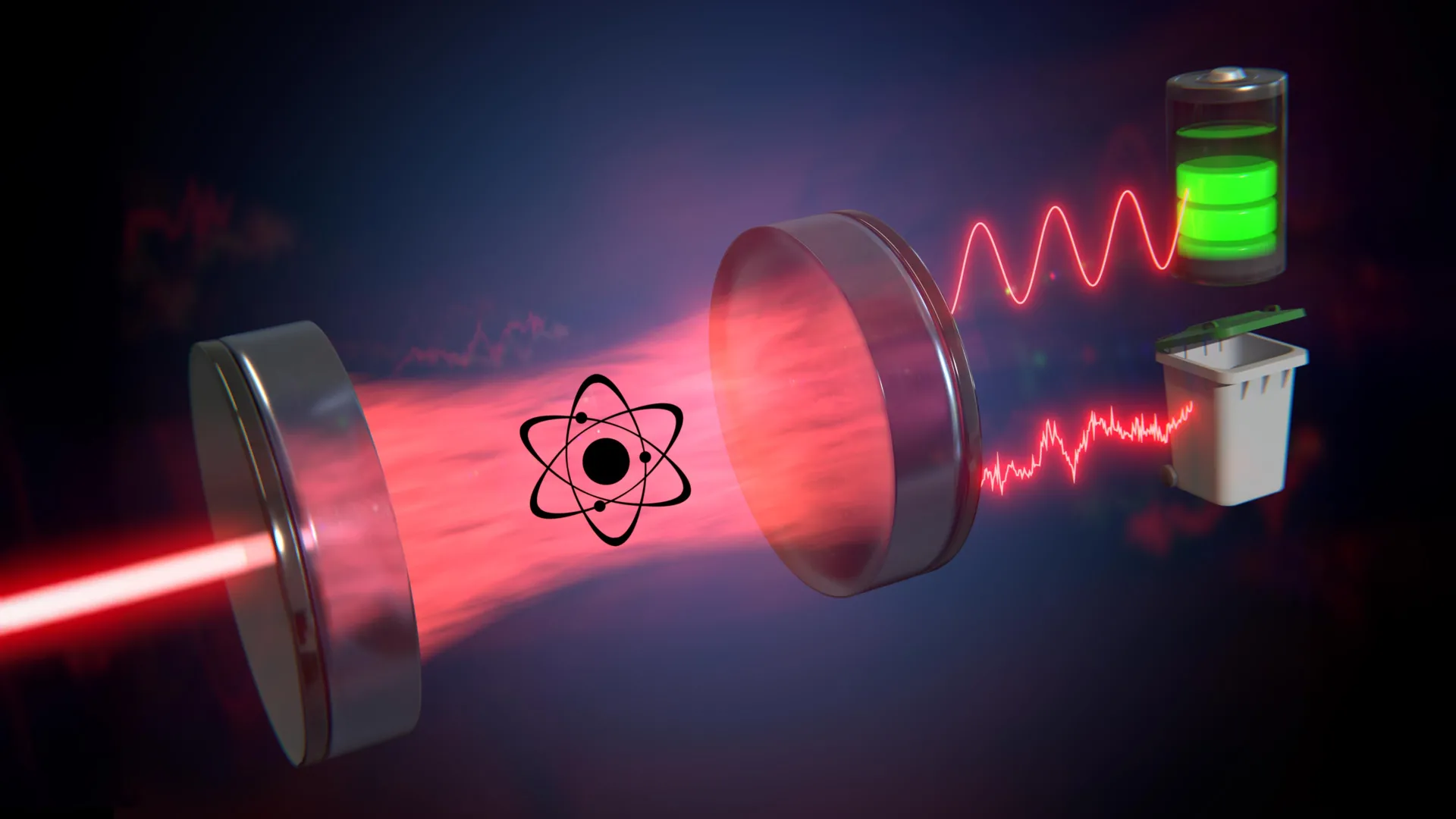
To explore this challenge, the team studied cavity resonators. These systems trap laser light between two mirrors, causing the light to bounce back and forth before some of it eventually escapes.
Laser light differs from the light produced by bulbs or LEDs because its electromagnetic waves move in perfect sync. When laser light travels through a cavity filled with atoms, this synchronization, known as coherence, can be disrupted. As a result, the light may become partly or fully incoherent (which corresponds to the disordered motion of particles). "The coherence of the light in such a laser-cavity-system was the starting point of our calculations," says Max Schrauwen, a bachelor's student involved in the study.
Work by Coherence
The researchers began by clarifying what "work" means for laser light. One example is the ability to charge a so-called quantum battery, which requires coherent light that can collectively push atoms into an excited state. A simple assumption would be that the incoming coherent light performs work, while the outgoing light, having lost some coherence, represents heat.
But the situation is more subtle. Even light that has become partially incoherent can still perform useful work, just less effectively than fully coherent light. Daniel and his colleagues examined what happens if only the coherent portion of the exiting light is counted as work, while the incoherent portion is treated as heat. With this definition, both laws of thermodynamics remain valid, showing that the framework is self-consistent.
Implications for Quantum Technology
"In the future, we can use our formalism to consider more subtle problems in quantum thermodynamics," says Daniel. This approach could prove valuable for emerging quantum technologies, including quantum networks. It may also help scientists better understand how familiar classical behavior emerges from the underlying quantum world.
Story Source:
Materials provided by University of Basel. Note: Content may be edited for style and length.
Journal Reference:
- Max Schrauwen, Aaron Daniel, Marcelo Janovitch, Patrick P. Potts. Thermodynamic Framework for Coherently Driven Systems. Physical Review Letters, 2025; 135 (22) DOI: 10.1103/zdbv-rksc
Cite This Page: