A simple oxygen hack creates 7 new ceramic materials
- Date:
- December 4, 2025
- Source:
- Penn State
- Summary:
- Penn State researchers created seven new high-entropy oxides by removing oxygen during synthesis, enabling metals that normally destabilize to form rock-salt ceramics. Machine learning helped identify promising compositions, and advanced imaging confirmed their stability. The method offers a flexible framework for creating materials once thought impossible to synthesize.
- Share:
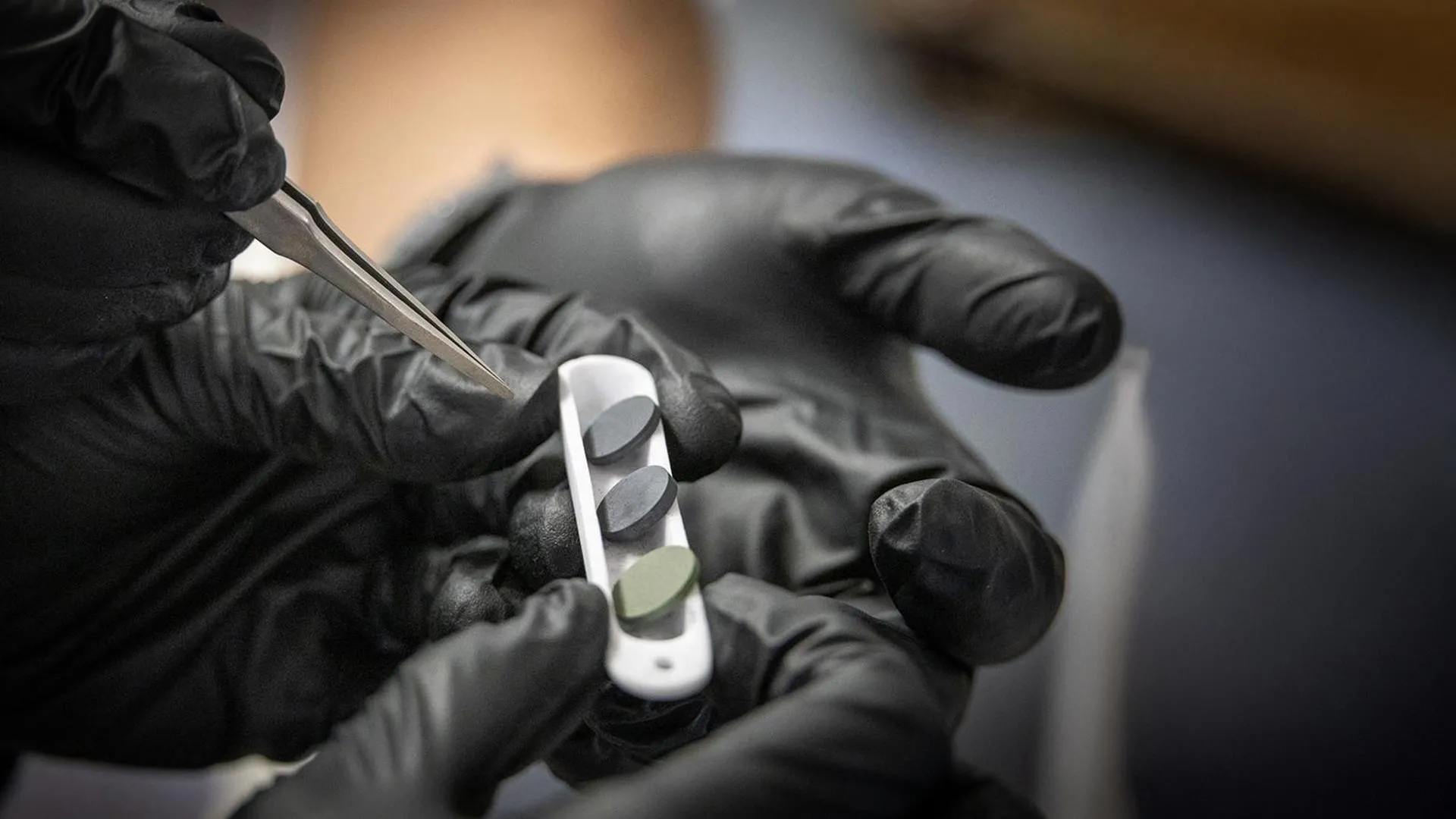
Sometimes, having less oxygen truly makes a difference. By lowering oxygen levels during synthesis, a group of materials scientists at Penn State succeeded in creating seven previously unknown high-entropy oxides, or HEOs. These ceramics contain five or more metals and are being explored for uses in energy storage, electronic devices and protective coatings.
During the development of these materials, the team also outlined a broader framework for designing future materials. Their findings were published in Nature Communications.
"By carefully removing oxygen from the atmosphere of the tube furnace during synthesis, we stabilized two metals, iron and manganese, into the ceramics that would not otherwise stabilize in the ambient atmosphere," said corresponding and first author Saeed Almishal, a research professor at Penn State working with Jon-Paul Maria, the Dorothy Pate Enright Professor of Materials Science.
Early Breakthroughs and Machine Learning Discovery
Almishal first achieved stability in a material containing manganese and iron by adjusting oxygen levels in a composition he designated as J52. That sample included magnesium, cobalt, nickel, manganese and iron. After that initial success, he used newly developed machine learning capabilities that can rapidly evaluate thousands of possible formulations. With those tools, he identified six additional metal combinations capable of forming HEOs.
Working alongside undergraduate researchers who helped process, fabricate and characterize samples, Almishal produced solid ceramic pellets representing all seven new HEO compositions. These students were supported by the Department of Materials Science and Engineering and Penn State's Center for Nanoscale Science, a U.S. National Science Foundation-funded Materials Research Science and Engineering Center.
"In a single step, we stabilized all seven compositions that are possible given our current framework," Almishal said. "Although this was previously treated this as a complex problem in the field of HEOs, the solution was simple in the end. With a careful understanding of the fundamentals of material and ceramic synthesis science, and particularly the principles of thermodynamics, we found the answer."
How Oxygen Levels Shape the Materials
To stabilize these ceramics, manganese and iron atoms must remain in the 2+ oxidation state, forming what is known as a rock salt structure where each atom bonds with only two oxygen atoms. According to Almishal, this does not happen under typical oxygen-rich conditions. If synthesized in a normal atmosphere, manganese and iron would continue binding with oxygen and shift to a higher oxidation state, preventing the material from forming correctly. Reducing the oxygen in the tube furnace limits how many oxygen atoms are available, allowing the desired rock salt structure to form.
"The main rule we followed in synthesizing these materials is the role that oxygen plays in stabilizing such ceramic materials," Almishal said.
Confirming Structure and Planning Future Experiments
To verify that manganese and iron truly remained in the intended oxidation state, Almishal collaborated with researchers at Virginia Tech. Their team used an advanced imaging approach that examines how atoms absorb X-rays. By studying the resulting data, they could confirm the oxidation states of individual elements and demonstrate that the materials were stable.
The next stage of work will involve testing the magnetic properties of all seven new HEOs. The researchers also hope to use the same thermodynamic principles for oxygen control to stabilize other types of materials that are currently difficult to synthesize.
"This paper, which has already been accessed online thousands of times, seems to resonate with researchers because of its simplicity," Almishal said. "Although we focus on rock salt HEOs, our methods provide a broad adaptable framework for enabling uncharted, promising chemically disordered complex oxides."
Undergraduate Recognition and Research Collaboration
Because of his significant contributions in the lab, co-author and undergraduate materials science and engineering major Matthew Furst was invited to present the findings at the American Ceramic Society's (ACerS) Annual Meeting with Materials Science and Technology 2025, which took place Sept. 28 through Oct. 1 in Columbus, Ohio. This invitation is typically extended to faculty or senior graduate students.
"I am so grateful for the opportunities that I have had on this project and to be involved in every step of the research and publication process," Furst said. "Being able to present this material to a broad audience as an invited talk reflects my involvement and the excellent guidance I have received from my mentors. It means a lot to me to develop important communication skills as an undergraduate student, and I look forward to pushing myself further in the future!"
Team Members and Support
In addition to Almishal, Maria and Furst, the Penn State research team included undergraduate students Joseph Petruska and Dhiya Srikanth; graduate students Yueze Tan and Sai Venkata Gayathri Ayyagari; and Jacob Sivak, who recently earned a doctorate in chemistry with a materials science focus. Faculty collaborators included Nasim Alem, professor of materials science and engineering; Susan Sinnott, professor of materials science and engineering and of chemistry; and Long-Qing Chen, the Hamer Professor of Materials Science and Engineering, professor of engineering science and mechanics and of mathematics.
From Virginia Tech, the co-authors were Christina Rost, assistant professor of materials science and engineering, and graduate student Gerald Bejger.
The Penn State Center for Nanoscale Science, an U.S. National Science Foundation-funded Materials Research Science and Engineering Center, provided support for this research.
Story Source:
Materials provided by Penn State. Note: Content may be edited for style and length.
Journal Reference:
- Saeed S. I. Almishal, Matthew Furst, Yueze Tan, Jacob T. Sivak, Gerald Bejger, Joseph Petruska, Sai Venkata Gayathri Ayyagari, Dhiya Srikanth, Nasim Alem, Christina M. Rost, Susan B. Sinnott, Long-Qing Chen, Jon-Paul Maria. Thermodynamics-inspired high-entropy oxide synthesis. Nature Communications, 2025; 16 (1) DOI: 10.1038/s41467-025-63567-z
Cite This Page: