New technology eliminates “forever chemicals” with record-breaking speed and efficiency
- Date:
- December 25, 2025
- Source:
- Rice University
- Summary:
- A new eco-friendly technology can capture and destroy PFAS, the dangerous “forever chemicals” found worldwide in water. The material works hundreds to thousands of times faster and more efficiently than current filters, even in river water, tap water, and wastewater. After trapping the chemicals, the system safely breaks them down and refreshes itself for reuse. It’s a rare one-two punch against pollution: fast cleanup and sustainable destruction.
- Share:
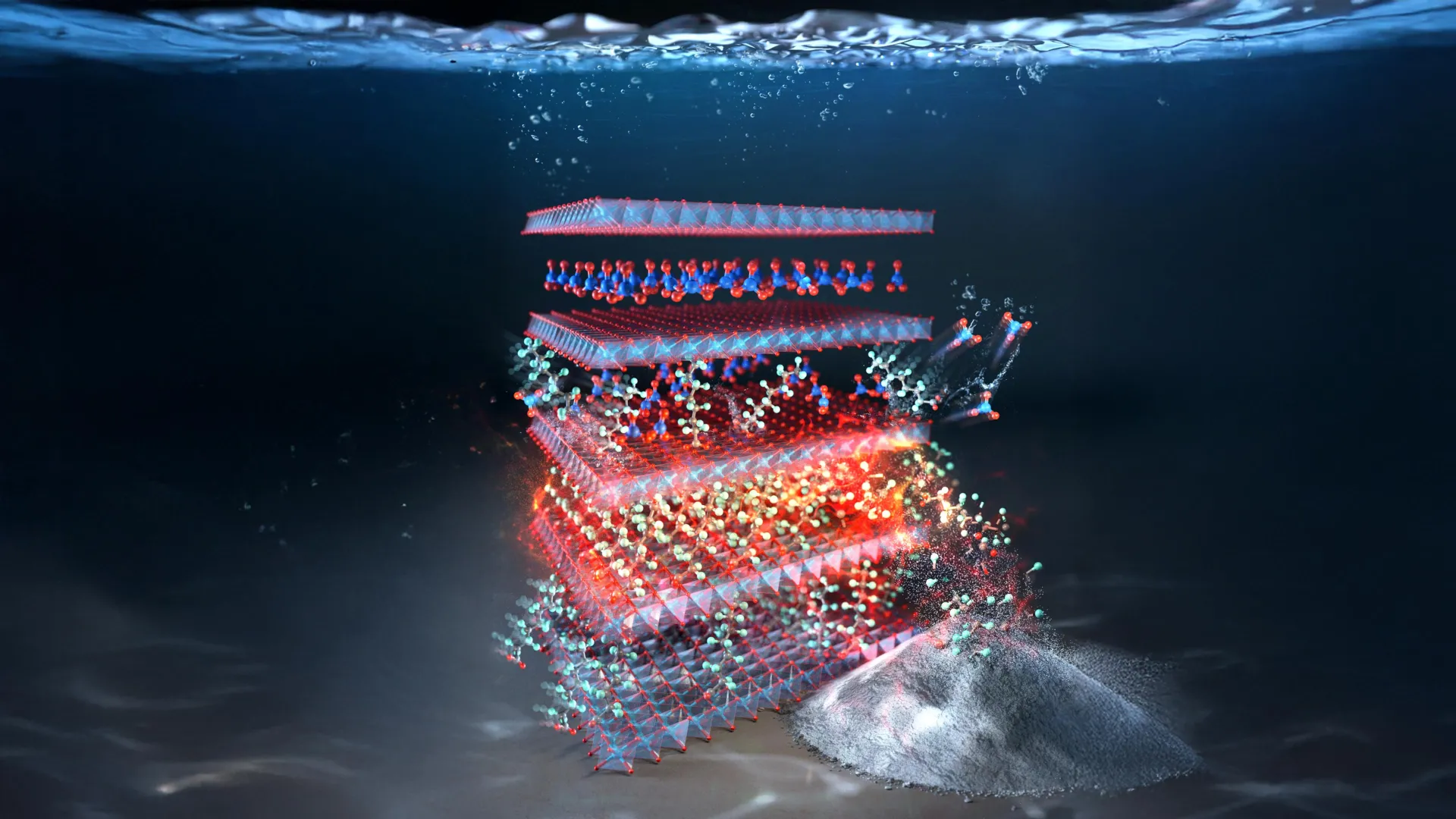
A research team at Rice University, working with international collaborators, has created the first environmentally friendly technology that can quickly trap and break down toxic "forever chemicals" (PFAS) in water. The results, published recently in Advanced Materials, represent a meaningful advance against one of the most stubborn pollution threats worldwide.
The project was led by Youngkun Chung, a postdoctoral fellow mentored by Michael S. Wong, a professor at Rice's George R. Brown School of Engineering and Computing. The effort also included Seoktae Kang, a professor at the Korea Advanced Institute of Science and Technology (KAIST), and Keon-Ham Kim, a professor at Pukyung National University in South Korea.
What Are PFAS and Why Are They a Problem
PFAS, short for per- and polyfluoroalkyl substances, are man-made chemicals that date back to the 1940s. They have been used in many everyday products, including Teflon pans, waterproof clothing, and some food packaging. PFAS became popular because they resist heat, grease, and water, but that same durability also means they break down very slowly, which is why they are often called "forever chemicals."
PFAS have now spread widely and can be found in water, soil, and air around the world. Research has linked exposure to liver damage, reproductive disorders, immune system disruption, and certain cancers. Cleanup has been difficult because once PFAS enter the environment, they are hard to remove and even harder to destroy.
Why Current PFAS Removal Methods Fall Short
Many standard approaches rely on adsorption, meaning the chemicals stick to materials such as activated carbon or ion-exchange resins. These methods are common, but they have major limitations, including low efficiency, slow operation, limited capacity, and the buildup of additional contaminated waste that still has to be handled.
"Current methods for PFAS removal are too slow, inefficient and create secondary waste," said Wong, the Tina and Sunit Patel Professor in Molecular Nanotechnology and professor of chemical and biomolecular engineering, chemistry and civil and environmental engineering. "Our new approach offers a sustainable and highly effective alternative."
The Breakthrough LDH Material That Works Fast
The new approach is built around a layered double hydroxide (LDH) material made from copper and aluminum. Kim first identified this type of material while he was a graduate student at KAIST in 2021. As the team explored these compounds further, Chung found that a specific version containing nitrate could adsorb PFAS with unusually high performance.
"To my astonishment, this LDH compound captured PFAS more than 1,000 times better than other materials," said Chung, a lead author of the study and now a fellow at Rice's WaTER (Water Technologies, Entrepreneurship and Research) Institute and Sustainability Institute. "It also worked incredibly fast, removing large amounts of PFAS within minutes, about 100 times faster than commercial carbon filters."
Researchers say the impressive results come from the material's internal design. Its ordered copper-aluminum layers, along with small charge imbalances, create a highly favorable surface where PFAS molecules can attach quickly and strongly.
Tested in River Water, Tap Water, and Wastewater
To see how well the system could perform outside the lab, the team tested the LDH material in river water, tap water, and wastewater. Across all three, it remained highly effective. It also performed well in both static tests and continuous-flow setups, pointing to possible use in municipal water treatment systems and industrial cleanup.
Closing the Loop With PFAS Destruction and Reuse
Capturing PFAS is only half the battle since the chemicals still need to be destroyed safely. Working with Rice professors Pedro Alvarez and James Tour, Chung developed a process to thermally decompose PFAS after they are captured on the LDH material. When the PFAS-loaded material was heated with calcium carbonate, the researchers removed more than half of the trapped PFAS without releasing toxic by-products. The same step also regenerated the LDH, making it possible to use the material again.
Early testing showed the material could go through at least six complete cycles of capture, destruction, and renewal. That makes it the first known eco-friendly, sustainable system for PFAS removal that combines rapid cleanup with repeated reuse.
International Collaboration and Research Support
"We are excited by the potential of this one-of-a-kind LDH-based technology to transform how PFAS-contaminated water sources are treated in the near future," Wong said. "It's the result of an extraordinary international collaboration and the creativity of young researchers."
A list of all the researchers involved in this study and their institutional affiliations can be found here. This research was supported by the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education (2021R1A6A3A14044449, RS-2023-00242795), grants from the National Convergence Research of Scientific Challenges and the Sejong Science Fellowship through the National Research Foundation of Korea and funding from the Ministry of Science (NRF-2022M3C1C8094245) and ICT (RS-2024-00395438). This work was also funded by Saudi Aramco-KAIST CO2 Management, Nanosystems Engineering Research Center for Nanotechnology-Enabled Water Treatment (NEWT), the U.S. Army Corps of Engineers' Engineering Research and Development Center grant (W912HZ-21-2-0050), Rice Sustainability Institute and Rice WaTER Institute.
Story Source:
Materials provided by Rice University. Note: Content may be edited for style and length.
Journal Reference:
- Keon‐Han Kim, Youngkun Chung, Philip Kenyon, Thi Nhung Tran, Nicholas H. Rees, Seung‐Ju Choi, Xiaopeng Huang, Jong Hui Choi, Phelecia Scotland, Sion Kim, Mohamed Ateia, Do‐Kyoung Lee, James M. Tour, Pedro J. J. Alvarez, Michael S. Wong, Seoktae Kang. Regenerable Water Remediation Platform for Ultrafast Capture and Mineralization of Per‐ and Polyfluoroalkyl Substances. Advanced Materials, 2025; DOI: 10.1002/adma.202509842
Cite This Page: