This tiny iron catalyst could transform the future of clean energy
- Date:
- August 27, 2025
- Source:
- Chinese Academy of Sciences Headquarters
- Summary:
- Hydrogen fuel cells could power cars, devices, and homes with nothing but water as a byproduct—but platinum’s cost holds them back. Chinese researchers have now unveiled a breakthrough iron-based catalyst that could rival platinum while boosting efficiency and durability. With its clever “inner activation, outer protection” design, this new catalyst not only reduces harmful byproducts but also shatters performance records, potentially paving the way for cleaner, cheaper, and more practical hydrogen energy.
- Share:
Proton exchange membrane fuel cells (PEMFCs), often referred to as "hydrogen power banks," are clean energy devices that generate electricity from hydrogen and oxygen with only water as a byproduct. Characterized by high efficiency, rapid start-up, and zero emissions, they hold great promise in transportation, portable electronics, and stationary power generation. Unfortunately, PEMFCs currently rely heavily on scarce and expensive platinum as a catalyst, making their widespread adoption impractical.
Now, however, a team of Chinese scientists has developed a high-performance iron-based catalyst for these fuel cells that could potentially reduce reliance on platinum. The new design, described as "inner activation, outer protection," enables record efficiency and long-term durability.
The findings were published in Nature.
Traditional Fe/N-C catalysts typically rely on outer surface of graphene or carbon supports, limiting the exposure of active sites and hindering their practical application. In general, PEMFCs have also been hampered by overly strong binding with oxygen intermediates, poor reaction kinetics, and vulnerability to Fenton reactions in oxidative environments (e.g., H2O2 and ·OH), leading to metal leaching and performance degradation.
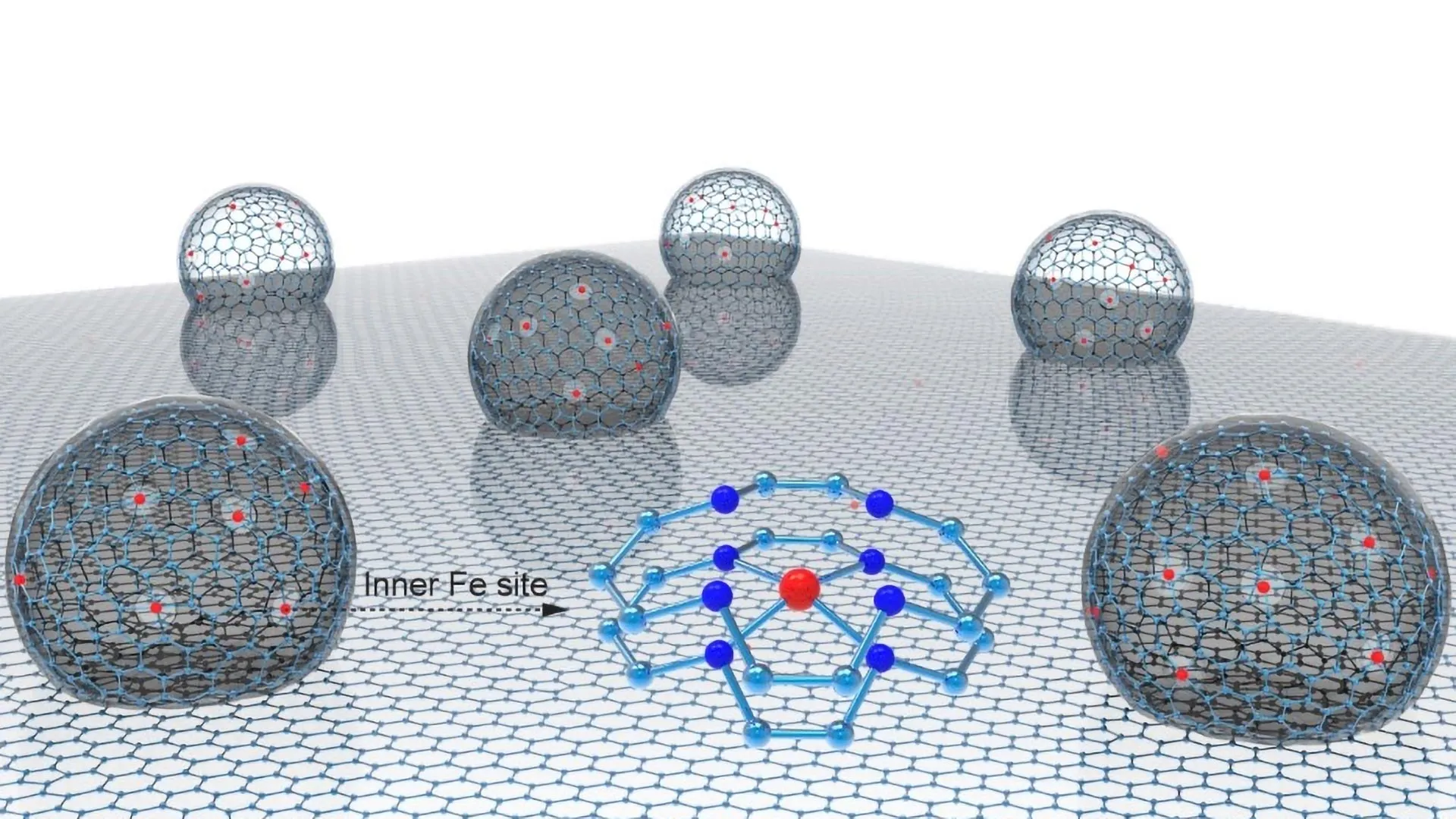
To address these challenges, the research team led by Prof. Dan Wang (currently at Shenzhen University) and Prof. ZHANG Suojiang from the Institute of Process Engineering of the Chinese Academy of Sciences developed an inner curved-surface single-atom iron catalyst (CS Fe/N-C) with a unique nanoconfined hollow multishelled structure (HoMS). Each nano hollow particle, about 10 nm × 4 nm in size, consists of multiple shells where Fe atoms are concentrated on the inner layers at high density.
This catalyst is composed of numerous nano HoMS dispersed on 2D carbon layers, with single-iron-atom sites primarily embedded within the inner curved surface of the nano HoMS. The outer graphitized carbon layer of the nano HoMS not only effectively weakens the binding strength of the oxygenated reaction intermediates but also reduces the hydroxyl radical production rate, forming a distinctive "inner activation, outer protection" microenvironment. The Fe/N-C catalyst delivers one of the best-performing platinum-group-metal-free PEMFCs.
Synchrotron X-ray absorption spectroscopy revealed that these inner Fe atoms predominantly exhibit a +2 oxidation state and an FeN4C10 coordination structure. Mössbauer spectroscopy further confirmed that 57.9% of the Fe sites are in a catalytically active low-spin D1 state.
Theoretical calculations showed that increasing curvature alone strengthens intermediate binding and hinders desorption, thereby reducing catalytic activity. However, introducing a nitrogen-doped carbon outer shell with Fe vacancies induces significant electrostatic repulsion (0.63-1.55 eV) between the outer-layer nitrogen atoms and the oxygen atoms of adsorbed intermediates on the inner shell. This repulsion weakens the binding strength, breaks the linear scaling relationship among ΔG*OH, ΔG*O, and ΔG*OOH, and significantly enhances the catalytic performance.
According to the researchers, the catalyst achieved an oxygen reduction overpotential as low as 0.34 V, which is far better than that of planar structure. It also suppressed hydrogen peroxide formation and improved selectivity and durability. Additionally, it delivered a record power density of 0.75 W cm-2 under 1.0 bar H2-air with 86% activity retention after more than 300 hours of continuous operation.
This work establishes a new type of CS Fe/N-C for highly active and durable oxygen reduction catalysis in fuel cells. The graphitized outer N-C layer effectively weakens the binding strength of oxygenated intermediates and suppresses ·OH generation, thereby improving both activity and stability. It provides a new paradigm for developing high-performance catalysts for next-generation electrocatalyst.
Story Source:
Materials provided by Chinese Academy of Sciences Headquarters. Note: Content may be edited for style and length.
Journal Reference:
- Yasong Zhao, Jiawei Wan, Chongyi Ling, Yanlei Wang, Hongyan He, Nailiang Yang, Rui Wen, Qinghua Zhang, Lin Gu, Bolong Yang, Zhonghua Xiang, Chen Chen, Jinlan Wang, Xin Wang, Yucheng Wang, Huabing Tao, Xuning Li, Bin Liu, Suojiang Zhang, Dan Wang. Acidic oxygen reduction by single-atom Fe catalysts on curved supports. Nature, 2025; 644 (8077): 668 DOI: 10.1038/s41586-025-09364-6
Cite This Page: