Scientists grow mini human livers that predict toxic drug reactions
A cutting-edge liver organoid platform reveals why some drugs harm only certain people, bringing personalized drug safety closer to reality.
- Date:
- October 15, 2025
- Source:
- Cincinnati Children's Hospital Medical Center
- Summary:
- A new human liver organoid microarray developed by Cincinnati Children’s and Roche recreates immune-driven liver injury in the lab. Built from patient-derived stem cells and immune cells, it accurately models how genetics influence drug reactions. The system replicated flucloxacillin-related toxicity seen only in people with a specific genetic variant, marking a major step toward predictive, patient-tailored drug safety testing.
- Share:
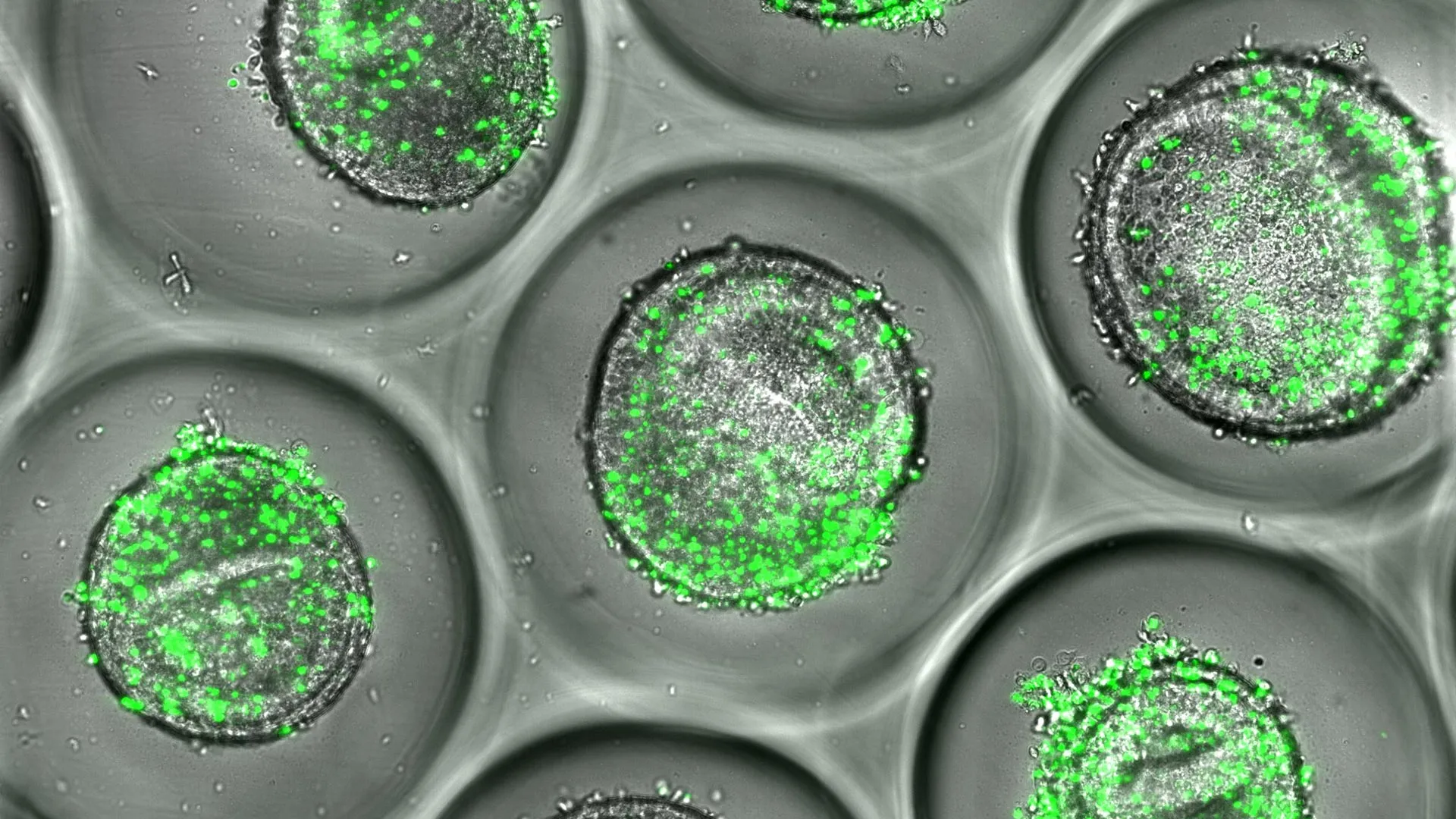
Researchers at Cincinnati Children's Hospital Medical Center, working in partnership with Roche, have created a next-generation human liver organoid microarray platform designed to predict which medications might trigger harmful immune responses in certain individuals.
The findings, published online on Sept. 26, 2025, in Advanced Science, describe a fully human, miniaturized liver system developed from stem cells and a patient's own immune cells. This advanced model provides a new way to study why some people suffer severe immune-related liver injuries from drugs that are otherwise considered safe. Co-first author Fadoua El Abdellaoui Soussi, PhD, and corresponding author Magdalena Kasendra, PhD, both from the Center for Stem Cell and Organoid Medicine (CuSTOM) at Cincinnati Children's, led the research.
"Our goal was to create a human system that captures how the liver and immune system interact in patients," El Abdellaoui Soussi says. "By integrating patient-specific genetics and immune responses, we can finally begin to explain why certain drugs cause liver injury in only a small subset of individuals."
A model that replicates immune-related liver injury
Certain drugs that pass traditional safety testing can still trigger idiosyncratic drug-induced liver injury (iDILI), a rare but serious immune reaction that can cause severe illness or force a drug to be withdrawn. Standard laboratory and animal models have long struggled to reproduce these complex immune responses that vary from person to person.
The new platform closes this gap by combining liver organoids made from induced pluripotent stem cells (iPSCs) with each donor's own CD8⁺ T cells, which are immune cells that target infected or damaged tissue. The result is a fully human, immune-competent system that reflects both the genetic and immune diversity found in real patients.
As proof of concept, the researchers recreated the liver damage caused by the antibiotic flucloxacillin, which occurs only in individuals who carry the HLA-B*57:01 risk gene. Their model accurately reproduced the biological signs of immune-related liver injury, including T cell activation, cytokine release, and liver cell damage, closely mirroring what happens in susceptible people.
"Our goal has always been to bring human biology into the lab in a way that's scalable, reproducible, and meaningful for patients," says Kasendra, who serves as director of research and development at CuSTOM. "By linking foundational stem cell science with applied toxicology, this model moves organoid research another step closer to transforming how drugs are developed and tested."
Building on a foundation of organoid innovation
This new platform expands on previous work by co-author Takanori Takebe, MD, PhD, whose lab developed methods for reliably generating human liver organoids from iPSCs. By refining these techniques into a matrix-free microarray system and pairing them with patient-specific immune cells, the CuSTOM Accelerator team at Cincinnati Children's turned a scientific breakthrough into a scalable precision toxicology tool.
The collaboration with Roche played a key role in the project's success, combining the hospital's scientific expertise with Roche's experience in translational toxicology.
"This partnership shows the power of combining academic innovation with industry experience," says Adrian Roth, PhD, principal scientific director of Personalized Healthcare Safety at Roche. "Together we're building predictive human models that can improve patient safety and accelerate the development of new medicines."
A growing ecosystem for organoid medicine
Cincinnati Children's has been a global leader in organoid medicine since 2010, when its scientists created the first functional human intestinal organoids.
Under Kasendra's leadership, the CuSTOM Accelerator partners with biopharma and technology companies to translate these scientific advances into real-world solutions for drug safety, precision medicine, and regenerative therapy.
What's next
The CuSTOM Accelerator team continues working to automate organoid assays and enable high-throughput screening across large, genetically diverse donor populations. This next phase will allow researchers to capture the full spectrum of human variability -- an essential step toward developing therapies that are more effective, inclusive, and personalized.
Learn more about CuSTOM's ongoing collaboration with Molecular Devices and Danaher: Collaboration to Develop Liver Organoids for Drug Toxicity Screening -- Research Horizons
"This work reflects the vision of CuSTOM -- to turn human organoid science into practical tools that improve health," Kasendra says, "This is just the beginning -- by bridging biology, engineering, and clinical insight, we're getting closer to predicting how real patients will respond to new treatments before they ever reach the clinic."
About the study
Cincinnati Children's and University of Cincinnati co-authors included co-first author Michael Brusilovsky, PhD, (now with Sanofi), Emma Buck, MS, (now at Imanis Life Sciences), W. Clark Bacon, MS, Sina Dadgar, PhD, Riccardo Barrile, PhD, and Michael Helmrath, MD. Collaborators also included experts from Genentech, Inc., and Molecular Devices LLC.
Funding sources for this research include Roche, Danaher, and the Farmer Family Foundation.
Story Source:
Materials provided by Cincinnati Children's Hospital Medical Center. Note: Content may be edited for style and length.
Journal Reference:
- Fadoua El Abdellaoui Soussi, Michael Brusilovsky, Emma Buck, W Clark Bacon, Sina Dadgar, Aaron Fullerton, Victoria Marsh Durban, Riccardo Barrile, Michael A. Helmrath, Takanori Takebe, Adrian Roth, Magdalena Kasendra. Autologous Organoid‐T Cell Co‐Culture Platform for Modeling of Immune‐Mediated Drug‐Induced Liver Injury. Advanced Science, 2025; DOI: 10.1002/advs.202508584
Cite This Page: